Reaction mechanisms in ionic liquids: the kinetics and mechanism of the reaction of O,O-diethyl (2,4-dinitrophenyl) phosphate triester with secondary alicyclic amines†
Abstract
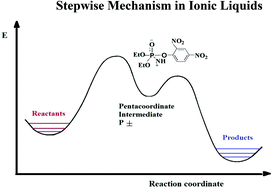
The reactions of O,O-diethyl 2,4-dinitrophenyl phosphate triester (1) with secondary alicyclic (SA) amines in the ionic liquids [Bmim]BF4 and [Bmim]DCA were subjected to a kinetic study. Eyring plots were obtained for the title reactions in the above ionic liquids (ILs) and also in aqueous ethanol (44 wt% ethanol). Two different reaction pathways were observed in [Bmim]BF4: nucleophilic attack at the phosphoryl center, SN2(P), and at the C-1 aromatic carbon, SN(Ar), where the product distribution remained constant and independent of the amine nature. In contrast, in [Bmim]DCA only the SN2(P) pathway was found. From the kinetic analysis of the SN2(P) pathway in both ILs, curved upwards plots of kobsdvs. 1-formylpiperazine concentration were obtained. Based on the kinetic behavior, a change in the mechanism of the SN2(P) pathway is proposed for the aminolysis of 1, from a concerted process in aqueous ethanol to a stepwise mechanism, through a zwitterionic pentacoordinate intermediate, when [Bmim]BF4 and [Bmim]DCA are used as the solvents of the reaction.

 Please wait while we load your content...
Please wait while we load your content...