Synthesis of two distinct pyrrole moiety-containing arenes from nitroanilines using Paal–Knorr followed by an indium-mediated reaction†
Abstract
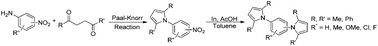
Synthesis of arenes substituted with two differently substituted-pyrrole moieties was investigated. A Paal–Knorr condensation reaction of nitroanilines with 1,4-diketone to nitrophenyl-1H-pyrroles followed by an indium-mediated reduction-triggered coupling reaction with another kind of 1,4-diketone resulted in two distinct pyrrole-containing arenes, variously substituted 1-((1H-pyrrol-1-yl)phenyl)-1H-pyrroles, in reasonable yield.

 Please wait while we load your content...
Please wait while we load your content...