Total synthesis of a piperidine alkaloid, microcosamine A†
Abstract
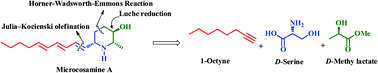
The first asymmetric total synthesis of a new natural piperidine alkaloid, microcosamine A, has been accomplished from D-serine and D-methyl lactate as chiral pool starting materials. Key features of the strategy include the utility of Horner–Wadsworth–Emmons reaction, Luche reduction, intramolecular carbamate N-alkylation to form the piperidine framework and Julia–Kocienski olefination to install the triene side-chain.

 Please wait while we load your content...
Please wait while we load your content...