Metal-free Brønsted acid mediated synthesis of fully substituted thiophenes via chemo- and regioselective intramolecular cyclization of α,α′-bis(β-oxodithioesters) at room temperature†
Abstract
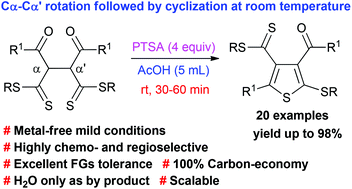
Metal-free para-toluenesulfonic acid mediated straightforward synthesis of hitherto unreported tetrasubstituted thiophenes has been achieved in quantitative yields by chemo- and regioselective dehydrative cyclization of α,α′-bis(β-oxodithioesters) at room temperature. Notably, the dithioester group at the 4-position of the thiophene ring has been further transformed to a thiazoline group.

 Please wait while we load your content...
Please wait while we load your content...