N-Bromosuccinimide promoted and base switchable one pot synthesis of α-imido and α-amino ketones from styrenes†
Abstract
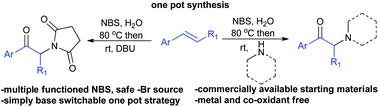
An N-Bromosuccinimide (NBS) promoted one pot strategy for the synthesis of α-amino functionalized aryl ketones starting from commercially available styrenes has been developed. NBS participates in multiple tasks, such as bromonium ion formation, oxidation of bromohydrin and providing a nucleophilic nitrogen source. The reaction can easily be switched between α-imido and α-amino ketones by the choice of base. This one pot strategy was successfully applied for the synthesis of psychoactive drug candidates, amfepramone, mephedrone and 4-MEC.

 Please wait while we load your content...
Please wait while we load your content...