Design and synthesis of simple, yet potent and selective non-ring-A pyripyropene A-based inhibitors of acyl-coenzyme A: cholesterol acyltransferase 2 (ACAT2)†
Abstract
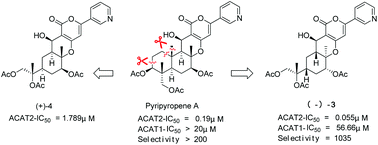
A series of pyripyropene A-based compounds were designed and synthesized by opening the upper section of the A-ring, which significantly simplifies the structure and synthesis from commercially available starting materials. Representative compound (−)-3 exhibited potent activity against ACAT2 and greater selectivity for ACAT2 than for ACAT1.

 Please wait while we load your content...
Please wait while we load your content...