An efficient synthesis of an exo-enone analogue of LL-Z1640-2 and evaluation of its protein kinase inhibitory activities†
Abstract
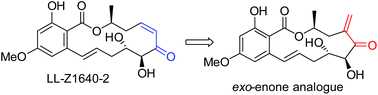
An efficient synthesis of an exo-enone analogue (5) of resorcylic acid lactone (RAL), natural product LL-Z1640-2 (1), has been achieved using a Ni-catalysed regioselective reductive coupling macrocyclisation of an alkyne–aldehyde as a key step. The synthetic route is significantly shorter than those for the natural product and avoids the isomerisation problem of the cis-double bond in the molecule. The preliminary biological evaluation showed that the exo-enone analogue is a potent inhibitor of several important kinases relevant to cancer drug development.

 Please wait while we load your content...
Please wait while we load your content...