Design, synthesis, biological evaluation and X-ray structural studies of HIV-1 protease inhibitors containing substituted fused-tetrahydropyranyl tetrahydrofuran as P2-ligands†
Abstract
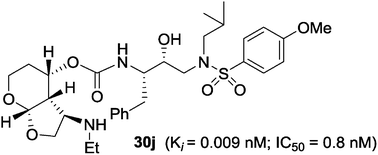
Design, synthesis, biological and X-ray crystallographic studies of a series of potent HIV-1 protease inhibitors are described. Various polar functionalities have been incorporated on the tetrahydropyranyl-tetrahydrofuran-derived P2 ligand to interact with the backbone atoms in the S2-subsite. The majority of the inhibitors showed very potent enzyme inhibitory and antiviral activity. Two high-resolution X-ray structures of 30b- and 30j-bound HIV-1 protease provide insight into ligand-binding site interactions. In particular, the polar functionalities on the P2-ligand appear to form unique hydrogen bonds with Gly48 amide NH and amide carbonyl groups in the flap region.

 Please wait while we load your content...
Please wait while we load your content...