Visible-light-promoted chloramination of olefins with N-chlorosulfonamide as both nitrogen and chlorine sources†
Abstract
A visible-light-promoted chloramination of olefins is reported. N-Chlorosulfonamides serve as both nitrogen and chlorine sources. These reactions provide a simple, efficient, regioselective, and atom-economical method for the preparation of vicinal haloamine derivatives under mild reaction conditions. A variety of olefins were tolerated, and chloramination products were obtained in good yields.
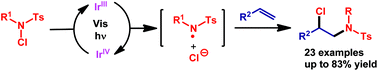

 Please wait while we load your content...
Please wait while we load your content...