The N-silylation of sulfoximines†
Abstract
The copper-catalyzed N-silylation of sulfoximines was achieved in the presence of di-tert-butyl peroxide. Notably, alkyl, phenyl and alkoxyl silanes were all suitable reaction partners. Mechanistic studies revealed that N-silyl acetamide serves as the intermediate.
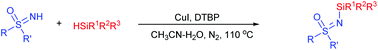

 Please wait while we load your content...
Please wait while we load your content...