Insights into the catalytic mechanism of synthetic glutathione peroxidase mimetics
Abstract
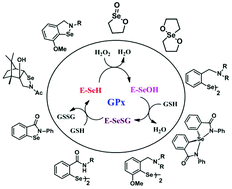
Glutathione Peroxidase (GPx) is a key selenoenzyme that protects biomolecules from oxidative damage. Extensive research has been carried out to design and synthesize small organoselenium compounds as functional mimics of GPx. While the catalytic mechanism of the native enzyme itself is poorly understood, the synthetic mimics follow different catalytic pathways depending upon the structures and reactivities of various intermediates formed in the catalytic cycle. The steric as well as electronic environments around the selenium atom not only modulate the reactivity of these synthetic mimics towards peroxides and thiols, but also the catalytic mechanisms. The catalytic cycle of small GPx mimics is also dependent on the nature of peroxides and thiols used in the study. In this review, we discuss how the catalytic mechanism varies with the substituents attached to the selenium atom.

 Please wait while we load your content...
Please wait while we load your content...