Synthesis of novel polysubstituted N-benzyl-1H-pyrroles via a cascade reaction of alkynyl Fischer carbenes with α-imino glycine methyl esters†
Abstract
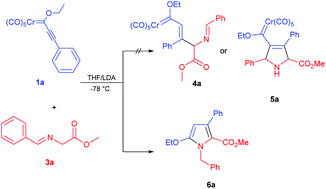
An efficient and simple synthesis of novel and densely substituted N-benzyl-1H-pyrroles 6a–r is described by a 1,4-addition/isomerization/ring closure/demetalation cascade process of alkynyl Fischer carbene complexes 1a–f and 2a and α-imino glycine methyl esters 3a, b, d, g, h, and k promoted with LDA.

 Please wait while we load your content...
Please wait while we load your content...