Late stage modification of receptors identified from dynamic combinatorial libraries†
Abstract
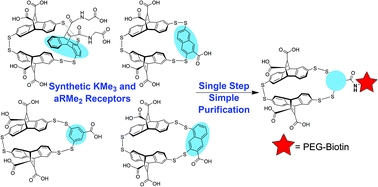
Small molecule receptors are attractive potential sensors of post-translational modifications, including methylated lysine and methylated arginine. Using dynamic combinatorial chemistry (DCC), our lab previously identified a suite of receptors that bind to Kme3 with a range of affinities ranging from low micromolar to high nanomolar, each with a unique selectivity for Kme3 over the lower methylation states. To enable these receptors to have broad application as Kme3 sensors, we have developed a method for their late-stage modification, which we used to synthesize biotinylated derivatives of A2B, A2D, and A2G in a single step. For our most attractive receptor for applications, A2N, we needed to develop an alternative method for its selective functionalization, which we achieved by “activating” the carboxylic acids on the constituent monomer A or N by pre-functionalizing them with glycine (Gly). Using the resulting Gly-A and Gly-N monomers, we synthesized the novel A2N variants A2Gly-N, Gly-A2N, and Gly-A2Gly-N, which enabled the late stage biotinylation of A2N wherever Gly was incorporated. Finally, we performed ITC and NMR binding experiments to study the effect that carboxylate spacing has on the affinity and selectivity of A2Gly-N and Gly-A2N for KmeX guests compared to A2N. These studies revealed the proximity of the carboxylates to play a complex role in the molecular recognition event, despite their positioning on the outside of the receptor.

 Please wait while we load your content...
Please wait while we load your content...