Synthesis and photophysical properties of pyrene-labeled 3-deaza-2′-deoxyadenosines comprising a non-π-conjugated linker: fluorescence quenching-based oligodeoxynucleotide probes for thymine identification†
Abstract
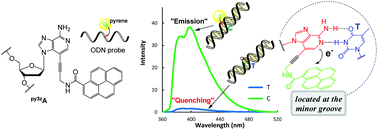
Pyrene-labeled 3-deaza-2′-deoxyadenosine comprising a non-π-conjugated linker py3zA (1) was synthesized and its photophysical properties were investigated. Oligodeoxynucleotide (ODN) probes containing py3zA (1) exhibited remarkable fluorescence quenching only when the opposite base of the complementary strand was the perfectly matched thymine. Such fluorescence quenching-based ODN probes exhibited excellent on-off switching properties, making them useful tools for single nucleotide polymorphism (SNP) genotyping and for the identification of target genes and structural studies of nucleic acids.

 Please wait while we load your content...
Please wait while we load your content...