Irreversible electron attachment – a key to DNA damage by solvated electrons in aqueous solution†
Abstract
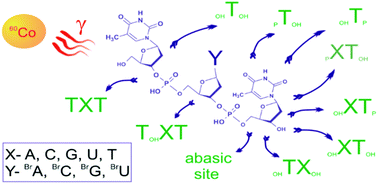
The TYT and TXT trimeric oligonucleotides, where X stands for a native nucleobase, T (thymine), C (cytosine), A (adenine), or G (guanine), and Y indicates a brominated analogue of the former, were irradiated with ionizing radiation generated by a 60Co source in aqueous solutions containing Tris as a hydroxyl radical scavenger. In the past, these oligomers were bombarded with low energy electrons under an ultra-high vacuum and significant damage to TXT trimers was observed. However, in aqueous solution, hydrated electrons do not produce serious damage to TXT trimers although the employed radiation dose exceeded many times the doses used in radiotherapy. Thus, our studies demonstrate unequivocally that hydrated electrons, which are the major form of electrons generated during radiotherapy, are a negligible factor in damage to native DNA. It was also demonstrated that all the studied brominated nucleobases have a potential to sensitize DNA under hypoxic conditions. Strand breaks, abasic sites and the products of hydroxyl radical attachment to nucleobases have been identified by HPLC and LC-MS methods. Although all the bromonucleobases lead to DNA damage under the experimental conditions of the present work, bromopyrimidines seem to be the radiosensitizers of choice since they lead to more strand breaks than bromopurines.

 Please wait while we load your content...
Please wait while we load your content...