Unsymmetrical 1,1-diborated multisubstituted sp3-carbons formed via a metal-free concerted-asynchronous mechanism†
Abstract
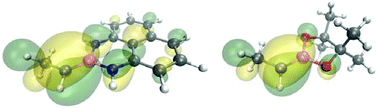
We have experimentally proved the unsymmetrical 1,1-diboration of diazo compounds, formed in situ from aldehydes and cyclic and non-cyclic ketones, in the absence of any transition metal complex. The heterolytic cleavage of the mixed diboron reagent, Bpin–Bdan, and the formation of two geminal C–Bpin and C–Bdan bonds has been rationalised based on DFT calculations to occur via a concerted-asynchronous mechanism. Diastereoselection is attained on substituted cyclohexanones and DFT studies provide understanding on the origin of the selectivity. The alkoxide-assisted selective deborylation of Bpin from multisubstituted sp3-carbon and generation of a Bdan stabilized carbanion, easily conducts a selective protodeboronation sequence.

 Please wait while we load your content...
Please wait while we load your content...