Conformational promiscuity in triazolamers derived from quaternary amino acids mimics peptide behaviour†
Abstract
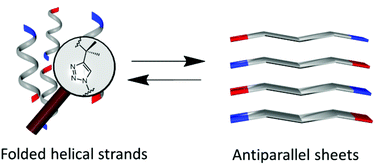
1,4-Substituted triazole oligomers made from quaternary amino acid derivatives present a conformational behaviour with similarities to that of natural peptides. Twisted strand and zig-zag type conformations have both been obtained in the crystal state. In a solution signs of weakly ordered structures have been detected depending on the substituents and the solvents used.

 Please wait while we load your content...
Please wait while we load your content...