Metal-free aerobic one-pot synthesis of substituted/annulated quinolines from alcohols via indirect Friedländer annulation†
Abstract
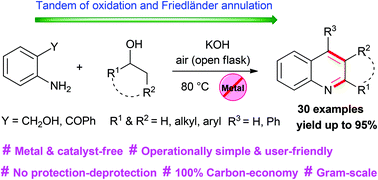
Metal-free, operationally simple, and highly efficient one-pot aerobic process for the synthesis of functionalized/annulated quinolines is devised from easily available 2-aminobenzyl alcohol/2-aminobenzophenones and alkyl/aryl alcohols for the first time. The process involves two sequential reactions, namely in situ aerial oxidation of alcohols to the corresponding aldehydes/ketones followed by Friedländer annulation.

 Please wait while we load your content...
Please wait while we load your content...