Stable analogues of nojirimycin – synthesis and biological evaluation of nojiristegine and manno-nojiristegine†
Abstract
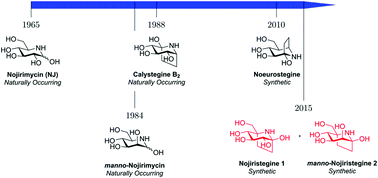
Two novel iminosugars called nojiristegines, being structural hybrids between nor-tropane alkaloid calystegine and nojirimycins, have been synthesised and found to be stable molecules despite the presence of a hemiaminal functionality. The synthesised iminosugars were evaluated against a panel of glycosidases and the best inhibition (IC50), found against α-glucosidases, was in the micromolar region. The compounds were also evaluated as potential antibiotics but no useful level of activity was observed.

 Please wait while we load your content...
Please wait while we load your content...