Aryne generation vs. Truce-Smiles and fries rearrangements during the Kobayashi fragmentation reaction: a new bi-aryl synthesis†
Abstract
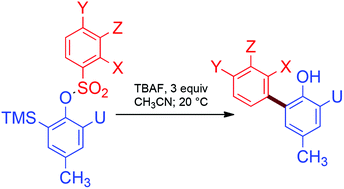
Treatment of (ortho-trimethysilyl)aryl phenylsulfonates with a soluble fluoride source initiates a Truce-Smiles rearrangement leading to the formation of functionalized bi-aryls. This new carbon–carbon bond-forming reaction proceeds without recourse to transition metal catalysis, under mild reaction conditions and with good functional group compatibility.

 Please wait while we load your content...
Please wait while we load your content...