Q2DSTD NMR deciphers epitope-mapping variability for peptide recognition of integrin αvβ6†
Abstract
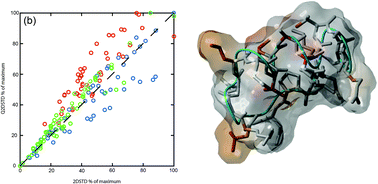
Integrin αvβ6 is a cell surface arginine-glycine-aspartic acid (RGD)-specific heterodimeric glycoprotein that is only expressed on epithelia during processes of tissue remodelling, including cancer. The specificity and molecular nature of interactions toward this integrin are poorly understood and new insights into such processes are important to cell biologists and pharmaceutical drug discovery. This study demonstrates the application of quantitative two-dimensional saturation transfer (Q2DSTD) NMR to obtain precise details of peptide interactions with integrin αvβ6 and their correlation to specificity for the integrin. This approach highlights subtle but significant differences in ligand contact by three related 21-mer peptides: FMDV2, an αvβ6 specific peptide and DBD1 and LAP2T1 peptides that bind many αv integrins in addition to αvβ6. FMDV2 and DBD1 differ only by the cyclisation of DBD1; a process that removes αvβ6 specificity. Q2DSTD NMR demonstrates these peptides experience significantly different interactions with the integrin; FMDV contacts primarily through four residues: 6Leu, 10Leu, 12Val and 13Leu, whereas DBD1 and LAP2T1 have more widespread contacts across their sequences. Q2DSTD NMR combined two-dimensional STD with quantitation by considering the relaxation of the ligand (CRL) to provide precise ligand contact information. This study also examines the role of CRL in the Q2DSTD process and how quantitation modifies STD data and unravels epitope-mapping variability to provide precise results that differentiate interactions at the atomic level for each peptide.

 Please wait while we load your content...
Please wait while we load your content...