N-heterocyclic carbene–palladium(ii)-1-methylimidazole complex catalyzed α-arylation reactions of tetralones with aryl chlorides and further transformation of the products†
Abstract
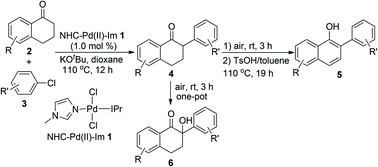
NHC–Pd(II)–Im complex 1 has proven to be an efficient catalyst in the reaction between tetralones 2 and aryl chlorides 3, giving the α-arylated tetralones 4 in good to high yields. In addition, if the above reaction mixture was exposed to air at room temperature for another 3 h, the normal α-arylated products 4 can be fully oxidized to 2-aryl-2′-hydroxytetralones 6 in good yields in a one-pot procedure. Furthermore, if the reaction mixture containing the oxidized products 6 was treated with TsOH/toluene solution under reflux for 19 h, the final aromatized products, 2-aryl-naphthalen-1-ols 5 can be achieved in acceptable to moderate yields. All reactions can tolerate various substituents on the tetralones 2 and aryl chlorides 3, thus giving an efficient method for the α-arylation of tetralones and further transformation of the products, and also enriching the applications of NHC–Pd(II) complexes in organic synthesis.

 Please wait while we load your content...
Please wait while we load your content...