An efficient microwave-assisted synthesis of cotinine and iso-cotinine analogs from an Ugi-4CR approach†
Abstract
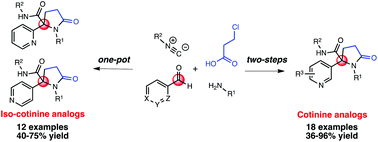
A convenient base-mediated two-step synthesis of cotinine analogs and a one-pot base-free synthesis of iso-cotinine derivatives featuring an Ugi-4CR/cyclization protocol are reported. These approaches exploit the reactivity of the peptidyl position present in the Ugi adducts, allowing the facile construction of the γ-lactam core, as well as the introduction of a N-substituted methyl group into the analogs in a straightforward manner. A plausible mechanism for the cyclization step is discussed.

 Please wait while we load your content...
Please wait while we load your content...