Multicomponent domino reactions of hydrazinecarbodithioates: concise access to 3-substituted 5-thiol-1,3,4-thiadiazolines†
Abstract
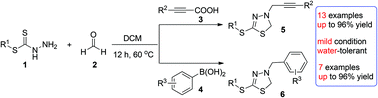
Two classes of addition/cycloaddition cascade reactions of hydrazinecarbodithioate (1) have been developed under mild reaction conditions. Reaction of hydrazinecarbodithioate (1) with formaldehyde solution (2) and propiolic acid (3) gives 3-propargyl-5-thiol-2,3-dihydro-1,3,4-thiadiazoles (5) via a decarboxylative coupling/cycloaddition domino sequence. When propiolic acid (3) is switched to phenyl boronic acid (4), a petasis/cycloaddition domino reaction is instead observed, in which 3-benzyl-5-thiol-2,3-dihydro-1,3,4-thiadiazoles (6) are obtained. Both these reactions show a wide range of functional-group compatibility for propiolic acids and aryl boronic acids, and give the corresponding products in moderate to good yields.

 Please wait while we load your content...
Please wait while we load your content...