Rhenium-catalyzed C–H aminocarbonylation of azobenzenes with isocyanates†
Abstract
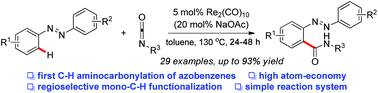
The first C–H aminocarbonylation of azobenzenes with isocyanates is achieved by using rhenium-catalysis, which provides an expedient and atom-economical access to varied o-azobenzamides from readily available starting materials. The reaction efficiency can be enhanced by the catalytic use of sodium acetate via accelerated C–H bond activation.

 Please wait while we load your content...
Please wait while we load your content...