One-pot synthesis of spiropyrroloquinoline-isoindolinone and their aza-analogs via the Ugi-4CR/metal-free intramolecular bis-annulation process†
Abstract
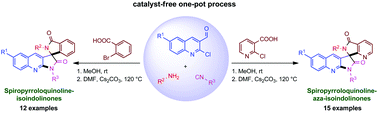
This presentation discloses a one-pot synthesis of a series of spiropyrroloquinoline isoindolinone and spiropyrroloquinoline aza-isoindolinone scaffolds. The reaction proceeds by the combination of a Ugi four-component reaction (4CR) and two intramolecular cyclizations under metal-free conditions. The proof of the structures relies on analytical investigation and X-ray crystallography.

 Please wait while we load your content...
Please wait while we load your content...