The first proton sponge-based amino acids: synthesis, acid–base properties and some reactivity†
Abstract
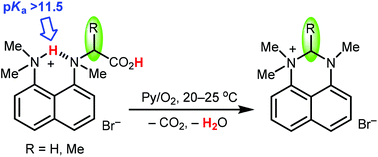
The first hybrid base constructed from 1,8-bis(dimethylamino)naphthalene (proton sponge or DMAN) and glycine, N-methyl-N-(8-dimethylamino-1-naphthyl)aminoacetic acid, was synthesised in high yield and its hydrobromide was structurally characterised and used to determine the acid–base properties via potentiometric titration. It was found that the basic strength of the DMAN–glycine base (pKa = 11.57, H2O) is on the level of amidine amino acids like arginine and creatine and its structure, zwitterionic vs. neutral, based on the spectroscopic (IR, NMR, mass) and theoretical (DFT) approaches has a strong preference to the zwitterionic form. Unlike glycine, the DMAN–glycine zwitterion is N-chiral and is hydrolytically cleaved with the loss of glycolic acid on heating in DMSO. This reaction together with the mild decarboxylative conversion of proton sponge-based amino acids into 2,3-dihydroperimidinium salts under air-oxygen was monitored with the help of the DMAN–alanine amino acid. The newly devised amino acids are unique as they combine fluorescence, strongly basic and redox-active properties.

 Please wait while we load your content...
Please wait while we load your content...