Synthesis and evaluation of galacto-noeurostegine and its 2-deoxy analogue as glycosidase inhibitors†
Abstract
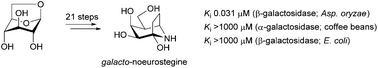
An epimer of the known glycosidase inhibitor noeurostegine, galacto-noeurostegine, was synthesised in 21 steps from levoglucosan and found to be a potent, competitive and highly selective galactosidase inhibitor of Aspergillus oryzae β-galactosidase. Galacto-noeurostegine was not found to be an inhibitor of green coffee bean α-galactosidase, yeast α-glucosidase and E. coli β-galactosidase, whereas potent but non-competitive inhibition against sweet almond β-glucosidase was established. The 2-deoxy-galacto-noeurostegine analogue was also prepared and found to be a less potent inhibitor of the same enzymes.

 Please wait while we load your content...
Please wait while we load your content...