A novel protocol for the facile construction of tetrahydroquinoline fused tricyclic frameworks via an intramolecular 1,3-dipolar nitrile oxide cycloaddition reaction†
Abstract
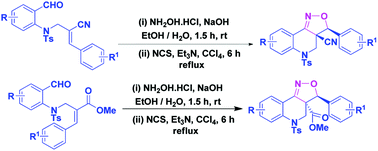
An efficient method towards the synthesis of quinoline fused tricyclic compounds involving an intramolecular 1,3-dipolar nitrile oxide cycloaddition reaction utilizing Baylis–Hillman derivatives in good yields has been described for the first time. A highly functionalized tricyclic framework was created by forming two rings and two adjacent stereocentres through the formation of two N–C bonds, one C–C bond and one O–C bond in a highly regio and diastereoselective manner.

 Please wait while we load your content...
Please wait while we load your content...