A new route for the synthesis of highly substituted 4-aminoquinoline drug like molecules via aza hetero–Diels–Alder reaction†
Abstract
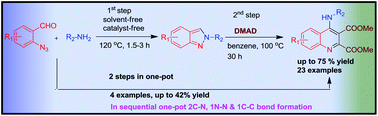
We have demonstrated for the first time the use of 2H-indazole as a diene partner in an aza hetero–Diels–Alder reaction leading to highly substituted 4-aminoquinolines via a heterocylic–heterocylic (H–H) strategy and have further developed a tandem one-pot method starting from 2-azidobenzaldehyde by the formation of 2C–N, 1N–N and 1C–C bonds. The key features of the present protocol are readily available starting materials, mild reaction conditions, being work-up free and having easy purification. This is the first time where the synthesis of 4-aminoquinoline drug like molecules has been achieved starting from simple starting materials in an atom economical manner.


 Please wait while we load your content...
Please wait while we load your content...