Highly stereoselective construction of novel dispirooxindole–imidazolidines via self-1,3-dipolar cyclization of ketimines†
Abstract
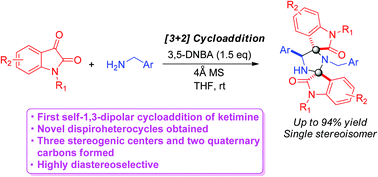
An acid-promoted self-1,3-dipolar cycloaddition of ketimines derived from isatins and benzylamines was successfully developed to assemble unprecedented dispirooxindole–imidazolidine ring systems. Generally, excellent diastereoselectivities (only a single stereoisomer formed) and good yields (up to 94%) were obtained. Consequently, this self-1,3-dipolar cycloaddition protocol offers facile access to a novel dispiroheterocyclic skeleton.

 Please wait while we load your content...
Please wait while we load your content...