Isoxazolidine-fused meso-tetraarylchlorins as key tools for the synthesis of mono- and bis-annulated chlorins†
Abstract
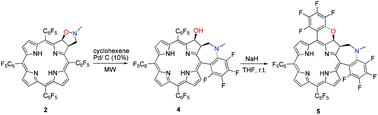
The microwave-assisted catalytic hydrogenation of the isoxazolidine-fused meso-tetrakis(pentafluorophenyl)chlorin afforded directly a mono-annulated chlorin with a singular 1-methyl-2,3-dihydro-1H-benzo[b]azepine ring that resulted from the cleavage of the isoxazolidine N–O bond followed by an intramolecular nucleophilic aromatic substitution of an o-F atom. The subsequent treatment of the mono-annulated chlorin with NaH induced a second intramolecular nucleophilic aromatic substitution, generating a bis-annulated chlorin having an additional 2H-pyran ring.

 Please wait while we load your content...
Please wait while we load your content...