Organocatalytic asymmetric intramolecular aza-Henry reaction: facile synthesis of trans-2,3-disubstituted tetrahydroquinolines†
Abstract
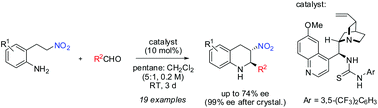
An enantio- and diastereoselective organocatalytic intramolecular aza-Henry (nitro-Mannich) reaction has been developed. The trans-2-aryl-3-nitro-tetrahydroquinoline products are obtained in high yields and in good enantioselectivities with a bifunctional tertiary amine-thiourea catalyst. Excellent enantioselectivities were obtained after single recrystallization of some products.

 Please wait while we load your content...
Please wait while we load your content...