Direct synthesis of a geminal zwitterionic phosphonium/hydridoborate system – developing an alternative tool for generating frustrated Lewis pair hydrogen activation systems†
Abstract
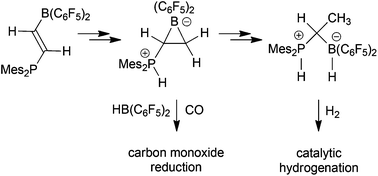
A convenient way to a new class of geminal Mes2PH+/B(C6F5)2H− pairs is presented. It utilizes triflic acid addition to trans-Mes2PCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHB(C6F5)2 followed by triflate/hydride exchange. Thermally induced ring-closure gave a phosphonium/boratacyclopropane zwitterion 8 which formed the Mes2PH(CHMe)B(C6F5)2H P/B FLP-H2 product 10 by subsequent treatment with triflic acid and a silane, or alternatively with dihydrogen at 90 °C. The product 10 is an active catalyst for the hydrogenation of a variety of unsaturated organic substrates, including a quinoline derivative. Treatment of compound 8 with HB(C6F5)2 gave a bifunctional borane 14 which selectively reduced carbon monoxide to the formyl stage.
CHB(C6F5)2 followed by triflate/hydride exchange. Thermally induced ring-closure gave a phosphonium/boratacyclopropane zwitterion 8 which formed the Mes2PH(CHMe)B(C6F5)2H P/B FLP-H2 product 10 by subsequent treatment with triflic acid and a silane, or alternatively with dihydrogen at 90 °C. The product 10 is an active catalyst for the hydrogenation of a variety of unsaturated organic substrates, including a quinoline derivative. Treatment of compound 8 with HB(C6F5)2 gave a bifunctional borane 14 which selectively reduced carbon monoxide to the formyl stage.

 Please wait while we load your content...
Please wait while we load your content...