The origin of the anomeric effect: probing the impacts of stereoelectronic interactions
Abstract
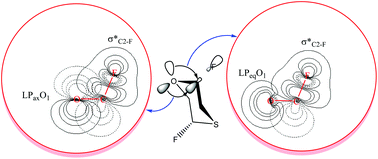
To gain further insight on the origin of the anomeric effect [stabilization energies associated with electron delocalization (SE), electrostatic models associated with the dipole–dipole interactions (EM) and Pauli exchange-type repulsions (PETR)], the correlations between SE, EM, PETR, bond-orders, donor and acceptor orbital energies and occupancies, structural parameters and configurational behavior of 2,3-difluoro-1,4-oxathiane (1), 2,3-dichloro-1,4-oxathiane (2), and 2,3-dibromo-1,4-oxathiane (3) as well as 2,5-difluoro-1,4-oxathiane (4), 2,5-dichloro-1,4-oxathiane (5), and 2,5-dibromo-1,4-oxathiane (6) were investigated by means of the complete basis set (CBS-4), hybrid density functional theory method (B3LYP/6-311+G**) and natural bond orbital (NBO) interpretations. The differences in the total energies among four possible configurations of compounds 1–6 do not correlate with the differences in their corresponding SE, EM or PETR values but can be controlled by their cooperative or uncooperative impacts. The results obtained showed that the SE has a determining impact on the structural properties of compounds 1–6 but fails to account solely for the variations of the energy differences between the configurations in compounds 1–6. The SE and PETR components are in favor of the (ax,ax) forms (the most stable configuration) going from compound 1 to compound 3 but the EM has the opposite impact; therefore, these factors have counterintuitive impacts on the configurational properties of compounds 1–3. Because there are no significant dipole moment values for the (ax,ax) and (eq,eq) forms of compounds 4–6, the energy differences between these forms can result from the conflict between the SE and PETR components. Therefore, the conclusions published previously in the literature about the origin of the anomeric effect should be reexamined.

 Please wait while we load your content...
Please wait while we load your content...