Photo-triggered fluorescent theranostic prodrugs as DNA alkylating agents for mechlorethamine release and spatiotemporal monitoring†
Abstract
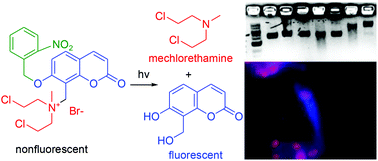
We describe a new theranostic strategy for selective delivery and spatiotemporal monitoring of mechlorethamine, a DNA alkylating agent. A photo-responsive prodrug is designed and composed of a photolabile o-nitrophenylethyl group, a DNA alkylating mechlorethamine drug and a coumarin fluorophore. Masking of the “N” in mechlorethamine in a positively charged state in the prodrug renders it inactive, non-toxic, selective and non-fluorescent. Indeed, the stable prodrug shows negligible cytotoxicity towards normal cells with and without UV activation and is completely non-fluorescent. However, upon photo-irradiation, the active mechlorethamine is released and induces efficient DNA cross-links, accompanied by a strong fluorescence enhancement (152 fold). Furthermore, DNA cross-linking activity from the release can be transformed into anticancer activity observed in in vitro studies of tumor cells. Importantly, the drug release progress and the movement can be conveniently monitored by fluorescence spectroscopy. The mechanistic study proves that the DNA cross-linking activity is mainly due to the release of DNA alkylating mechlorethamine. Altogether, the studies show the power of the theranostic strategy for efficient therapy in cancer treatment.

 Please wait while we load your content...
Please wait while we load your content...