Stereoselective reaction of 2-carboxythioesters-1,3-dithiane with nitroalkenes: an organocatalytic strategy for the asymmetric addition of a glyoxylate anion equivalent†
Abstract
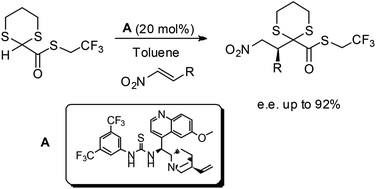
An efficient organocatalytic methodology has been developed to perform the stereoselective addition of 2-carboxythioesters-1,3-dithiane to nitroalkenes. Under mild reaction conditions γ-nitro-β-aryl-α-keto esters with up to 92% ee were obtained, realizing a formal catalytic stereoselective conjugate addition of the glyoxylate anion synthon. The reaction products are versatile starting materials for further synthetic transformations; for example, the simultaneous reduction of the nitro group and removal of the dithiane ring was accomplished, allowing the preparation of a GABAB receptor agonist baclofen.

 Please wait while we load your content...
Please wait while we load your content...