A mechanistic investigation of anti-elimination in (Z)-1,2-bis(arylseleno)-1-alkenes and their sulfur analogs†
Abstract
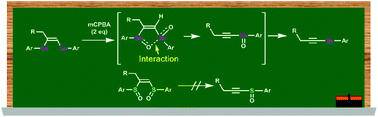
The oxidation of (Z)-1,2-bis(arylseleno)-1-alkenes is known to afford alkynyl selenoxides via a unique selenoxide anti-elimination mechanism; however, to date, there have been no mechanistic studies of this reaction. During our studies of this transformation, monoselenoxides 6 and 7 were unexpectedly isolated as stable reaction intermediates. In addition, 77Se NMR studies of the reaction mixture revealed the presence of an intramolecular Se⋯O interaction and the formation of alkynyl selenoxides. Meanwhile, even at higher temperatures, the reaction of a (Z)-1,2-bis(arylsulfinyl)-1-alkene, the sulfur analog of (Z)-1,2-bis(arylseleninyl)-1-alkenes, did not proceed via sulfoxide elimination but proceeded via isomerization and disproportionation. Therefore, the intramolecular Se⋯O interaction can be concluded to play a pivotal role in the anti-elimination reaction.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST

 Please wait while we load your content...
Please wait while we load your content...