A GGCT fluorogenic probe: design, synthesis and application to cancer-related cells†
Abstract
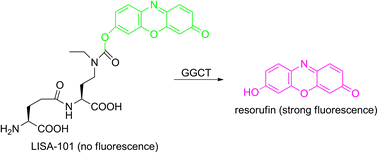
Cancer-related γ-glutamyl cyclotransferase (GGCT) specifically converts γ-glutamyl amino acids (γ-Glu-Xaa) into pyroglutamate and the corresponding amino acids (Xaa). Here we report a novel GGCT fluorogenic probe “LISA-101” containing a masked O-acylated fluorophore “resorufin” on the side chain of the P′1 amino acid (Xaa). Upon GGCT treatment, the P′1 amino acid was liberated and spontaneously released the intact fluorophore. Thus, the fluorescence was regained. LISA-101 will expand the strategies for cancer studies.


 Please wait while we load your content...
Please wait while we load your content...