Formamidine hydrochloride as an amino surrogate: I2-catalyzed oxidative amidation of aryl methyl ketones leading to free (N–H) α-ketoamides†
Abstract
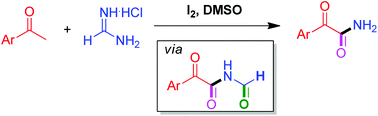
A highly efficient molecular iodine catalyzed oxidative amidation of aryl methyl ketones with formamidine hydrochloride has been developed. This reaction represents a novel strategy for the synthesis of free (N–H) α-ketoamides. Based on the experimental results, a self-sequenced iodination/Kornblum oxidation/amidation/oxidation/decarbonylation mechanism was proposed.

 Please wait while we load your content...
Please wait while we load your content...