Synthesis and biological studies of the thiols-triggered anticancer prodrug for a more effective cancer therapy†
Abstract
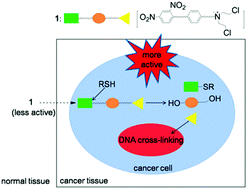
A novel anticancer prodrug compound 1, which was designed to be triggered by thiols and release the chemotherapeutic agent mechlorethamine, was successfully prepared and evaluated for the first time. The activation of compound 1 was determined by NMR analysis and denaturing alkaline agarose gel electrophoresis. A fluorescence image and comet assay indicated that the inducible reactivity of 1 could be accomplished in cell media. The anticancer activities are also discussed.

 Please wait while we load your content...
Please wait while we load your content...