Conformational modulation of peptides using β-amino benzenesulfonic acid (SAnt)†
Abstract
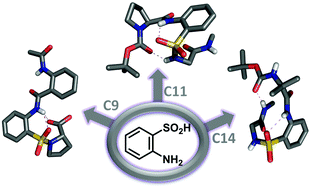
This communication describes the utility of a conformationally restricted aromatic β-amino acid (2-aminobenzenesulfonic acid, SAnt) inducing various folding interactions in short peptides. Sandwiching SAnt between diverse amino acid residues was shown to form robust folded architectures featuring a variety of H-bonded networks, suggesting its utility in inducing peptide folding.
 Please wait while we load your content...
Please wait while we load your content...