Palladium-catalyzed oxidative deacetonative coupling of 4-aryl-2-methyl-3-butyn-2-ols with H-phosphonates†
Abstract
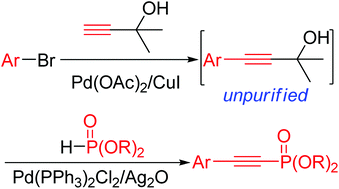
An efficient and generally applicable protocol for palladium-catalyzed oxidative deacetonative coupling of 4-aryl-2-methyl-3-butyn-2-ols with dialkyl H-phosphonates has been developed. This methodology provides a new and practical route to alkynylphosphonates using the inexpensive 4-aryl-2-methyl-3-butyn-2-ols as the alkyne sources. This reaction could also be performed with aryl bromides, 2-methyl-3-butyne-2-ol and dialkyl H-phosphonates using the cheap 2-methyl-3-butyne-2-ol as an alkyne source.

 Please wait while we load your content...
Please wait while we load your content...