P-stereogenic PNP pincer-Pd catalyzed intramolecular hydroamination of amino-1,3-dienes†
Abstract
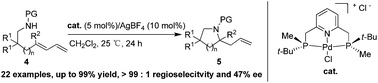
A new P-stereogenic PNP pincer-Pd complex was readily prepared from optically pure 2,6-bis[(boranato(tert-butyl)methylphosphino)methyl]pyridine. It was used in the asymmetric intramolecular hydroamination of amino-1,3-dienes, with the desired products being obtained in good yields and with excellent regioselectivities and up to moderate enantioselectivities. The absolute configuration of one of the hydroamination products was determined by X-ray crystallography studies. This simple and efficient procedure can be used for the synthesis of allyl-type chiral pyrrolidine derivatives.

 Please wait while we load your content...
Please wait while we load your content...