Chemoselective efficient synthesis of functionalized β-oxonitriles through cyanomethylation of Weinreb amides†
Abstract
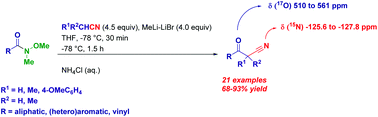
A synthesis of β-oxonitriles is reported via the generation of R1R2CLiCN species followed by the trapping with variously decorated Weinreb amides. The optimization study revealed that lithiation of acetonitriles is best accomplished by deprotonation with MeLi–LiBr at low temperature. The protocol can be conveniently adapted to the synthesis of α-mono or α,α-disubstituted cyanoketones. 15N- and 17O-NMR data are reported for selected compounds.
 Please wait while we load your content...
Please wait while we load your content...