Synthesis of 1- and 4-substituted piperazin-2-ones via Jocic-type reactions with N-substituted diamines†
Abstract
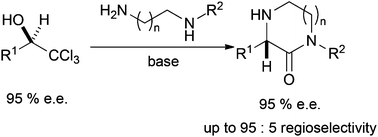
Enantiomerically-enriched trichloromethyl-containing alcohols, obtained by asymmetric reduction, can be transformed regioselectively into 1-substituted piperazinones by modified Jocic reactions with little or no loss of stereochemical integrity. This methodology can be easily used to synthesise important pharmaceutical compounds such as the fluorobenzyl intermediate of a known PGGTase-I inhibitor.

 Please wait while we load your content...
Please wait while we load your content...