Direct oxidative coupling of thiols and benzylic ethers via C(sp3)–H activation and C–O cleavage to lead thioesters†
Abstract
An unprecedented C–S formation method via direct oxidative C(sp3)–H bond functionalization and C–O cleavage of benzylic ethers was developed. Various thioesters including thioester structure containing drug intermediates could be achieved by this convenient, metal and base free method in satisfactory yields.
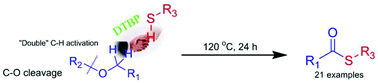
 Please wait while we load your content...
Please wait while we load your content...