Enantioselective synthesis of benzoindolizidine derivatives using chiral phase-transfer catalytic intramolecular domino aza-Michael addition/alkylation†
Abstract
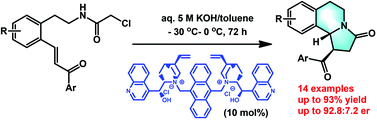
An efficient and enantioselective strategy to synthesize benzoindolizidines from α,β-unsaturated amino ketones via domino intramolecular aza-Michael addition/alkylation was developed. These reactions were enabled by cinchona alkaloid-derived quaternary ammonium salts as the phase-transfer catalyst. A variety of benzoindolizidines were prepared in good yields (up to 93%) and enantioselectivities (up to 92.8 : 7.2 er).

 Please wait while we load your content...
Please wait while we load your content...