An enantioselective total synthesis of Sch-725674†
Abstract
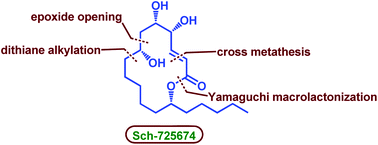
An enantioselective total synthesis of Sch-725674, a unique 14-membered macrolactone, has been accomplished in 13 steps. The step-wise dithiane alkylation served as a strategic step to assemble the upper and lower fragments of the molecule, whereas cross metathesis reaction, Yamaguchi macrolactonization and a substrate controlled stereoselective reduction are used as key steps to complete the total synthesis.

 Please wait while we load your content...
Please wait while we load your content...