From α-nucleophiles to functionalized aggregates: exploring the reactivity of hydroxamate ion towards esterolytic reactions in micelles†
Abstract
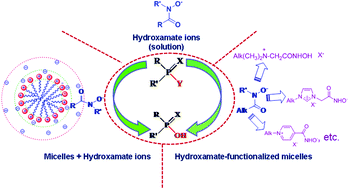
Owing to the rising threats of neurotoxic organophosphosphorus compounds, facile and efficient decontamination systems are required. Since the last few decades, the search for promising α-nucleophiles for straightforward and eco-friendly decontamination reactions using α-nucleophiles has been considerably boosted up. Among these, hydroxamic acids have been widely studied due to their potential α-nucleophilicity towards carbon and phosphorus based esters. This account summarizes our research on α-nucleophilicity of hydroxamate ions in water and micelles towards esterolytic reactions. Efforts of our group in the last few years have been collectively judged and compared with the crucial findings of researchers in the relevant field. The present article sheds light on the rich chemistry of the hydroxamate ion as a perfect candidate to degrade organophosphorus esters (i.e. nerve agents, pesticides and their simulants) in water, in micelles of conventional surfactants, and in functionalized micelles. The current report also provides an insight into the possible nature and mechanisms of these reactions. A brief account of the biological activities of hydroxamic acids that have recently spurred research in medicine against some fatal diseases has been included.
- This article is part of the themed collection: Recognition and Reactivity at Interfaces

 Please wait while we load your content...
Please wait while we load your content...